- Rf = Distance moved by the pigment / Distance moved by the solvent
Measure the distance in cm from the starting point (pencil line) to the center of each pigment band. Then measure the entire distance traveled by the solvent. Remember, the starting point for the solvent is also the pencil line and the ending point for the solvent is the top edge of the paper. Do the required divisions and record your Rf values in the DATA TABLE of your report sheet.
6. Wash the mortar and pestle thoroughly, using a little alcohol to remove any remaining pigment.
7. Repeat steps 1 through 6 for each species.
DATA TABLE:

Back to Biology Table of Contents
Back to Lab Dad's Laboratory
CHROMATOGRAPHY
INTRODUCTION:
Chlorophyll often hides the other pigments present in leaves. In Autumn, chlorophyll breaks down, allowing xanthophyll and carotene, and newly made anthocyanin, to show their colors.
The mix of pigments in a leaf may be separated into bands of color by the technique of paper chromatography. Chromatography involves the separation of mixtures into individual components. Chromatography means "color writing." With this technique the components of a mixture in a liquid medium are separated. The separation takes place by absorption and capillarity. The paper holds the substances by absorption; capillarity pulls the substances up the paper at different rates. Pigments are separated on the paper and show up as colored streaks. The pattern of separated components on the paper is called a chromatogram.
PRELAB PREPARATION:
Gather leaves from several different plants. CAUTION: Avoid poisonous plants. Autumn leaves from deciduous trees are especially interesting. Sort the leaves by kind (maple, etc.) and color. Review a diagram of a plant cell . Find the grana and the chloroplasts of the cell.
MATERIALS:
Safety goggles
Chromatography solvent (92 parts Petroleum ether to 8 parts acetone)
Chromatography paper (or filter paper) about 1 cm x 15 cm
Ethyl alcohol
Fresh spinach
Test tube
Test tube rack
Scissors and Ruler
Fresh leaves of plants
Glass stirring rod
Paper clip
Cork (to fit test tube)
Mortar and pestle
Sand (optional)
10-ml Graduated cylinder
PROCEDURE:
Leaves should be grouped by kind (maple, etc.) and color. Work with a spinach leaf and with one or more other types. CAUTION: Chromatography solvents are flammable and toxic. Have no open flames; maintain good ventilation; avoid inhaling fumes.
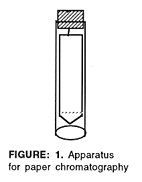
1. Cut a strip of filter paper or chromatography paper so that it just fits inside a 15-cm (or larger) test tube. Cut a point at one end. Draw a faint pencil line as shown in figure 1. Bend a paper clip and attach it to a cork stopper. Attach the paper strip so that it hangs inside the tube, as shown. The sides of the strip should not touch the glass.
2. Tear a spinach leaf into pieces about the size of a postagestamp. Put them into a mortar along with a pinch or two of sand to help with grinding. Add about 5 ml ethyl alcohol to the leaf pieces. Crush leaves with the pestle, using a circular motion, until the mixture is finely ground. The liquid in which the leaf pigments are now for paper chromatography dissolved is called the pigment extract.
3. Use a glass rod to touch a drop of the pigment extract to the center of the pencil line on the paper strip. Let it dry. Repeat as many as 20 times, to build up the pigment spot. NOTE: You must let the dot dry after each drop is added. The drying keeps the pigment dot from spreading out too much.
4. Pour 5 ml chromatography solvent into the test tube. Fit the paper and cork assembly inside. Adjust it so that the paper point just touches the solvent (but not the sides of the tube). The pigment dot must be above the level of the solvent. Watch the solvent rise up the paper, carrying and separating the pigments as it goes. At the instant the solvent reaches the top, remove the paper and let it dry. Observe the bands of pigment. The order, from the top, should be carotenes (orange), xanthophylls (yellow), chlorophyll a (yellow-green), chlorophyll b (blue-green), and anthocyanin (red). Identify and label the pigment bands on the dry strip. Write the species of leaf on the strip as well.
Record the species, external color, and chromatogram pigments in the DATA TABLE of your report sheet.
5. Each pigment has an Rf value, the speed at which it moves over the paper compared with the speed of the solvent.