AN INTRODUCTION TO THE METALLURGY OF STEEL
[ Home ] [ Table of Content ] [ Next ]
Pure iron is only slightly harder and stronger than copper. Its great ductility and formability are conducive to making hand art, but its low strength is not very practical for industrial engineering designs. With the addition of carbon to iron, steel is created, providing the strength required for modern engineering applications. Its mechanical properties rise to the occasion, limited only by the designer's imagination.
However, it is the phenomenon of allotropy in iron that yields the almost unlimited range of properties of steel. To our good fortune, allotropy in iron is retained even in the presence of other alloying elements in steel, allowing for many forms of heat treatable steel alloys to produce a variety of properties for various applications.
Iron, as shown in figure 1, exists in three crystal (atomic) allotropes, namely: alpha (a) iron, delta (d) iron, and gamma (g) iron. The a-iron
|
|
form exists below 1625oF (885oC) while d-iron is stable above 2540oF ( 1395oC). Gamma iron exists at the temperatures between these two ranges. It is the allotropy of iron that allows for these crystal structures to change with temperature.
At room temperature, the a-iron crystal structure has its atoms arranged in a geometric pattern known as body-centered cubic or bcc (figure 2) . This atomic arrangement of iron atoms is magnetic up to 1420oF (770oC), called the curie temperature. This temperature was of practical importance to the early blacksmiths who used an iron horseshoe magnet with a steel bar across the two ends for temperature measurement. When the steel bar fell from the magnet, the blacksmith knew the approximate temperature of the hearth and was able to adjust the heat treat schedule accordingly. Above the curie temperature is still bcc but is no longer magnetic. Slow heating of a-iron to 1625oF (885oC) produces an allotropic change to gamma (g) iron, a face-centered cubic (fcc) crystal structure which is non-magnetic (figure 2). A change from one crystal structure to another is called a transformation and the temperature at which it occurs is called the transformation temperature.
When fcc g- iron is slowly heated above 2540oF (1395oC) it transform back to bcc iron. To distinguish the elevated temperature bcc iron from its lower temperature counterpart, it is given its own name, delta (d) iron. This d-iron is non-magnetic and exists until the temperature is raised to 2800oF (1540oC) which causes melting of the solid d-iron to liquid iron. Since atoms in the liquid iron have no distinct arrangement (each atom moving freely within the liquid) there no longer exists a crystal structure above the melting temperature.
For the allotropic transformations descri bed,
there is another driving force equally important to the transformation
temperature, namely, time. For allotropic transformations to occur
at the temperatures suggested in figure 1, sufficient time is required for the
atoms to reorganize themselves in the new crystal structure. At the
lower end of the temperature ranges for each allotrope of iron, lower energy
levels exist, so more time is required for crystal structure transformation to
occur. The interaction of time and temperature to achieve the
allotropic transformations shown in figure 1 is called
equilibrium.
bed,
there is another driving force equally important to the transformation
temperature, namely, time. For allotropic transformations to occur
at the temperatures suggested in figure 1, sufficient time is required for the
atoms to reorganize themselves in the new crystal structure. At the
lower end of the temperature ranges for each allotrope of iron, lower energy
levels exist, so more time is required for crystal structure transformation to
occur. The interaction of time and temperature to achieve the
allotropic transformations shown in figure 1 is called
equilibrium.
Note in figure 1 that the heating/cooling curve flattens at the allotropic transformation temperatures. This pause in the heating/cooling cycle is necessary for equilibrium allotropic change in the crystal structures to occur. This form of graphical presentation was made popular by French scientists, and consequently, the transformation temperatures are designated by the letter A (from the French word arreter - meaning to stop), or r (from refroidir - meaning to cool). For example, Ac3 is transformation temperature of a-iron to g-iron upon heating and Ar4 is the transformation temperature of d-iron to g-iron upon cooling.
The allotropic transformations illustrated in figure 1 are reversible, such taht the transformations can occur upon slow heating or slow cooling. It is this powerful flexibility of iron that provides the opportunity to heat treat steels to many metallurgical conditions and associated mechanical and physical properties.
It becomes apparent that a clear understanding of the behaviour of iron is imperative in discussing steels and their many alloys. In this light, let's continue this discussion by alloying the iron with carbon to make steel.
THE IRON-IRON CARBIDE SYSTEM - SLOW COOLING
Carbon is the most significant alloying element in steel. One of
its most pronounced effects is on the transformation temperatures as shown in
figure 3. The addition of carbon to iron lowers the A3
temperature, while it raises the A4 temp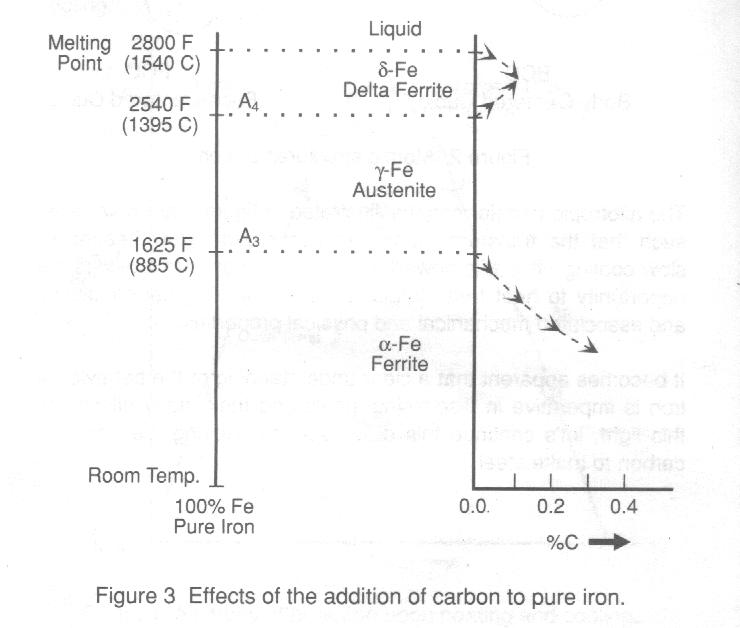 erature
and lowers the melting temperature. Expanding this diagram to display the
various allotropic crystal structure changes results in the classic iron-iron
carbide (Fe-Fe3C) phase diagram shown in figure 4.
Although this diagram may seem quite involved at first glance, it is a
relatively simple but powerful tool in understanding the metallurgy of steels.
erature
and lowers the melting temperature. Expanding this diagram to display the
various allotropic crystal structure changes results in the classic iron-iron
carbide (Fe-Fe3C) phase diagram shown in figure 4.
Although this diagram may seem quite involved at first glance, it is a
relatively simple but powerful tool in understanding the metallurgy of steels.
The area enclosed by QGPQ is a solid solution phase of carbon dissolved in alpha iron known as alpha (a) ferrite, more commonly called ferrite. Ferrite has a body-centered (bcc) crystal structure that can only dissolve a maximum of 0.025% C at 1340oF (725oC), with the solubility of carbon dropping to 0.008% C at room temperature, i.e. almost pure iron.
The term ferrite was first used by the American metallurgist Professor Henry M. Howe, and was almost certainly derived from the Latin word ferrum, meaning iron. Since the ferrite phase at room temperature is essentially pure iron, only containing 0.008% C, it has little commercial use because of its extreme softness and low strength. [ Go to Top ]
Delta iron, with carbon contents of up to 0.1% C, exists at temperatures
above 254 0oF
(1395oC) and is called delta (d)
ferrite. This area of the diagram becomes of importance to
welding when considering hot cracking in carbon and alloy steels, since
d-ferrite has relatively good solubility of sulphur; where sulphur is the
main cause of hot cracking. When the term ferrite is used, it is
understood that
a-ferrite is the subject
material. Likewise, when discussing the elevated temperature ferrite, one must
use the term delta ferrite.
0oF
(1395oC) and is called delta (d)
ferrite. This area of the diagram becomes of importance to
welding when considering hot cracking in carbon and alloy steels, since
d-ferrite has relatively good solubility of sulphur; where sulphur is the
main cause of hot cracking. When the term ferrite is used, it is
understood that
a-ferrite is the subject
material. Likewise, when discussing the elevated temperature ferrite, one must
use the term delta ferrite.
As a rule of thumb, steels with < 0.25% C are called low carbon or mild steels; steels with 0.25 - 0.50% C are called medium carbon steels; and steels with > 0.50% C are called high carbon steels.
The area in figure 4 enclosed by GJIEHG is a solid solution known as austenite. Austenite is a non-magnetic, face-centered cubic (fcc) crystal structure, that can dissolve carbon interstitially to a maximum of 2% at 2100oF (1150oC) and is exhibited schematically in figure 5. Austenite was first reported by Floris Osmond, a French steelworks engineer, and named by him in honour of the eminent English metallurgist, Professor Sir William C. Roberts-Austens.
When heat treat procedures involve heating steels in the region of Fe-Fe3C phase diagram, the term used to describe the heat treatment is austenizing. A steel is said to become austenitized when it has been heated at a sufficient temperature, for the appropriate time, to achieve 100% austenite through the thickness of the part.
At 6.67% carbon and room temperature, ferrite is no longer stable. Instead, the iron atoms combine with carbon atoms to form iron carbide (Fe3C), called cementite, existing within the boundary DOMD in figure 4. The crystal structure of cementite is orthorhombic.
The term cementite was first applied by Professor Howe and was probably
derived from cement carbon, refe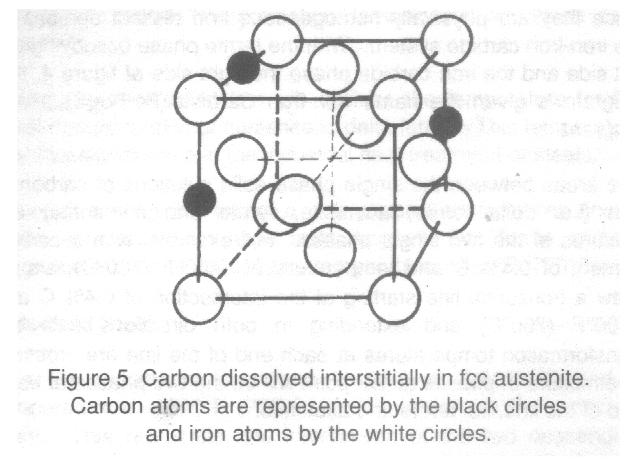 rring
to carbon which was introduced into steel at that time by the cementation
process. Like all carbides, cementite is an extremely hard
constituent. When place in a soft matrix of ferrite, its
distribution and size produce the extraordinary range of mechanical properties
that steel is noted for.
rring
to carbon which was introduced into steel at that time by the cementation
process. Like all carbides, cementite is an extremely hard
constituent. When place in a soft matrix of ferrite, its
distribution and size produce the extraordinary range of mechanical properties
that steel is noted for.
Delta ferrite, austenite, ferrite and cementite are called phases since they are physically homogeneous and distinct portions of the iro-iron carbide system. With the ferrite phase occupying the left side and the iron carbide phase the right side of figure 6, this diagram is given the name Iron-Iron Carbide (Fe-Fe3C) phase diagram.
The areas between the single phase solid solutions of carbon in iron (i.e. delta ferrite, austenite, ferrite and cementie) are mixtures of the two single hpases. For example, with a carbon content of 0.4% C and temperature of 1400oF (760oC), simply draw a horizontal line starting at the intersection of 0.4% C and 1400oF (760oC) and extending in both directions until the transformation temperatures of each end of the line are crossed. The mixture of phases at this point will be the two phases at each end of the line, i.e. ferrite and austenite. [ Go to Top ]
Therefore, from figure 4: ferrite plus cementite exists within the boundary QPNOQ; ferrite plus austenite exists within the boundary PGHP; delta ferrite plus austenite exists within the boundary JKIJ; delta ferrite plus liquid exists within the boundary KABK; austenite plus liquid exists within the boundary EIBCE; austenite plus cementite exists within the boundary HEMNH; and liquid plus cementite exists within the boundary CDMC.
Transformation Temperatures and Lines
The horizontal line PN extending along 1340oF (720oC) represents the lower critical temperature, and is the first transformation line reached upon heating steel from room temperature. It is designated as the A1 line.
The line GH defines the temperature at which complete transformation to austenite is achieved upon heating steel with up to 0.8% C. In steel heat treating terms, it is referred to as the upper critical temperature and is designated as A3. The line HE represents the Acm temperature that borders the lower limit of the austenitic region for steels with greater than 0.8% C. It becomes the upper critical transformation temperature for these high carbon steels.
The A4 transformation line (JI) outlines the temperature for the initial transformation of austenite to delta ferrite. This temperature has little significance in the industrial heat treatment of steels.
Although the A2 line is not a true phase transformation line, it does represent the change from magnetic bcc ferrite to non-magnetic bcc ferrite at the Curie temperature, 1420oF (770oC).
Point H represents a carbon content of 0.8% C and a temperature of 1340oF (725oC) and is known as the eutectoid point. Thhis represents the intersection of the two descending transformation lines, A3 and Acm, with the horizontal transformation line A1. Steel with this composition (0.8% C) is known as eutectoid steels.
Steels having a carbon content less than 0.8% C are called hypoeutectoid steels and those with more than 0.8% C are called hypereutectoid steels. (A simple reminder to keep track of these two terms is to remember that hyper rhymes with the word higher and thus hypereutectoid steel has the higher carbon content, i.e. > 0.8% C. By elimination, the other term, hypoeutectoid steel, has less than 0.8% C.)
When a eutectoid steel (0.8% C) is cooled slowly from an austenitizing temperature, say 1500oF (815oC), according to the Fe-Fe3C phase diagram, no transformation will occur until the temperature reaches the eutectoid temperature 1340oF (725oC). Upon further slow cooling below
 |
this temperature, austenite will transform to ferrite and cementite.
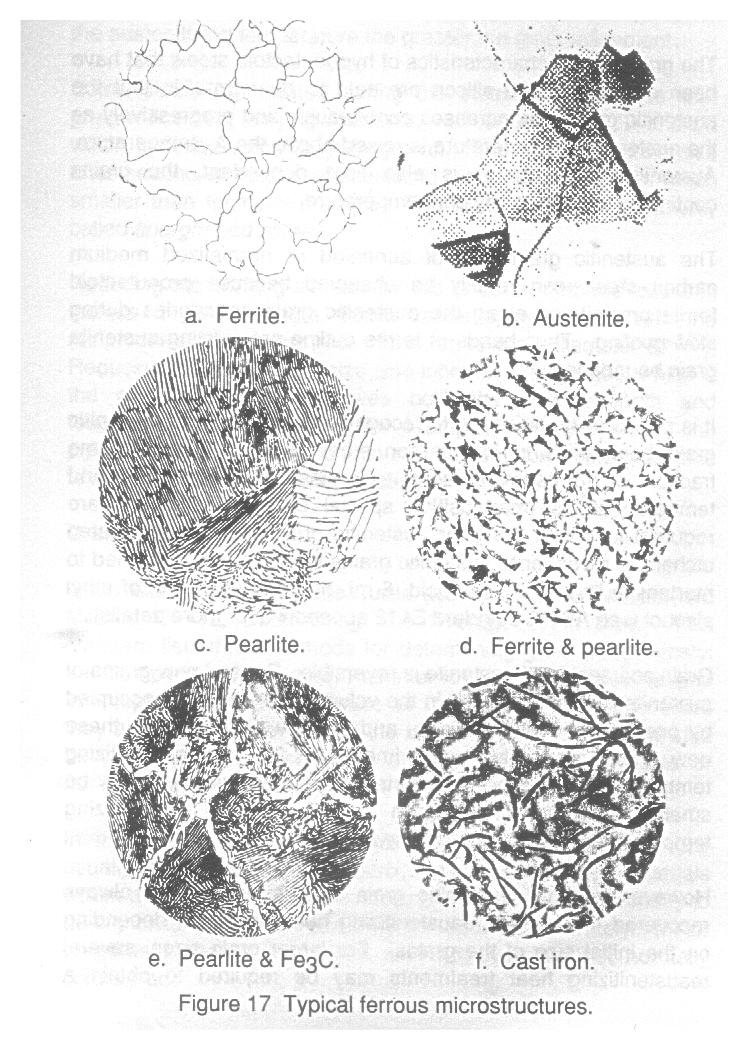
However, the transformation is unique since the carbon previously dissolved in the austenite cannot be retained by the newly formed ferrite, due to the low solubility of carbon in ferrite. Consequently, carbon is rejected by the new ferrite and accumulates as cementite laths (or layars) adjacent to ferrite layers as schematically represented in figure 6. The microstructure of alternating laths of ferrite and cementite is called Pearlite. Eutectoid steels (0.8% C), when slow cooled after austenitizing, will form 100% pearlite (figure 17c).
Pearlite was first observed by the 19th century English geologist, Dr. Henry C. Sorby, and was named pearlyte and later pearlite by Professor Howe. Its name is said to be derived from the shiny microscopic appearance resembling that of the mother-of-pearl. [ Go to Top ]
The width of the alternating laths of ferrite and cementite govern the mechanical properties of this microstructure. When pearlite is formed under very slow cooling, the pearlite laths are wider than if cooled under relatively faster rates. Pearlite containing wider laths is known as coarse pearlite and is softer and weaker microstructure than pearlite with narrower laths, called fine pearlite. It is important to remember that pearlite is not a phase of steel, but rather a microstructure made up of two phases, namely ferrite and cementite.
In terms of understanding the heat treatment of steels, the decomposition of austenite is paramount. Consider austenite in a hypoeutectoid steel of 0.4% C at 1550oF (843oC) and slow cooling (say 100oF/hr) to room temperature. The following observations can be made:
1. Above the A3 line, austenite is stable and can easily dissolve the 0.4% C into its fcc solid solution. Be aware that the higher the austenitizing temperature reached above the A3 line and/or the longer the time at the austenitizing temperature, the larger the austenite grain size will become. This is called grain growth.
2. Upon cooling the fcc austenite from 1550oF (843oC), it begins to transform to bcc ferrite at the A3 temperature, approximately 1475oF (802oC). This phase transformation of austenite to ferrite continues as we cool within PGHP (figure 4) region. Note that as the temperature is decreased within this region, more ferrite is formed at the expense of losing austenite. Since ferrite can dissolve no more than a maximum of 0.025% C, the carbon content of the remaining (untransformed) austenite is increased as proeutectoid (new) ferrite is formed. This continues until just above the A1 line where the remaining austenite will contain essentially 0.8% C.
3. At the A1 line, 1340oF (725oC), the remaining austenite begins its transformation to pearlite. As the A1 line is crossed, the remaining austenite transform to pearlite and the resultant microstructure is a mixture of ferrite and pearlite.
4. Cooling from just below the A1 line, where ferrite and pearlite are now present, produces no further phase changes. The room temperature microstructure will remain ferrite and pearlite.
Ferrite grain size and pearlite volume fraction are a key factor in determining low temperature impact toughness. The smaller the final grain size and the lower the pearlite amount, the higher the low temperature impact toughness will become.
Consider austenitizing a hypereutectoid steel of 1.0% C at 1550oF (843oC) followed by slow cooling (say 100oF/hr). The following observations can be made:
1. Above the Acm line, austenite is stable and can easily dissolve the 1.0% C. Again, the higher the temperature reached above the Acm line and/or the longer the time at that temperature, the larger the austenite grain will become. Also remember that to achieve an austenitizing condition, sufficient time at the austenitizing temperature is required to produce 100% austenite through the thickness of the steel part.
2. As the Acm temperature, about 1450oF (787oC), is met upon cooling, austenite begins to give up (called precipitation) some of its carbon, thus forming the new phase, cementite (Fe3C). The amount of austenite decreases as new cementite is formed, with decreasing temperature approaching the A1 line.
3. At the A1 line, 1340oF (72oC), sufficient carbon has been precipitated from the austenite solid solution that it now retains only 0.8% C. This is the eutectoid composition, and hence, the remaining austenite transforms to pearlite upon further cooling.
4. Once the A1 line has been crossed, the resultant microstructure consists of cementite and pearlite. Cementite is peresent within the pearlite or as a network around the pearlite grains (see figure 17c and e). There are no further phase changes as the steel cools to room temperature.
Eutectic and Eutectoid Reactions
It is important to distinguish between the eutectoid and eutectic reactions in the iron - carbon system. The eutectoid reaction at 0.8 and 1340oF (725oC) involves one solid solution phase (austenite) transforming on cooling to a mixture of two solid solution phases (ferrite + cementite). By comparison, the eutectic reaction at 4.3% C and 2100oF (1150oC) involves one liquid phase transforming on cooling into a mixture of two solid solution phases (ledeburite and cementite).
Fe-Fe3C Phase Diagram Reactions [ Go to Top ]
Industrial fabrication conditions restrict the application of the iron-iron carbide phase diagram, since:
1. Commercial additions of other elements (Mn, Si, Cr, Ni, Mo, etc.) shiftnthe position of the transformation lines, i.e. changing the transformation temperatures, with the extent of the change depending on the element and the amount added.
2. Faster rates of heating annd cooling, such as in welding and quenching, greatly exceed the equilibrium rates (i.e. slow cooling and cooling), so that the transformation reactions are shifted, delayed, or simply do not have sufficient time to occur.
However, the diagram can be used in many industrial heat treatment application of plain carbon steels and as a rough guide for alloy steels and when considering welding or any other thermal process.
THE IRON-IRON CARBIDE SYSTEM - FAST COOLING
If austenite is allowed to cool faster than the rates required to produce a ferrite-pearlite structure, then at temperstures below about 1025oF (550oC) another constituent, bainite starts to separate along with pearlite. At these faster cooling rates, the potential for austenite to transform to ferrite and pearlite is suppressed by the inability of the carbon atoms to move fast enough to their equilibrium positions. The main reason for this occurrence is the lack of heat-energy retained in the material with the faster cooling rates; remembering that sufficient time and tempersature (energy) is required for carbon atom diffusion to produce the ferrite-pearlite transformations from austenite.
In 1934 the term bainite was initiated to honour Edgar C. Bain by his colleagues at the Kearney Laboratory - Jose Vilella, John Zimmerman, E.S. Davenport, E.L. Roff and Robert Aborn, In fact, Bain and associates were not the first to produce the bainite microstructure, since Portevin had done so in 1911, but at that time it was impossible to interpret the phase with the existing technology. Bainite was formerly referred to by the now obsolete terms, sorbite and troostite.
Depending on the temperature of formation, bainite varies from a fine mixture of ferrite and cementite to lens-shaped needles of ferrite and no visible cementite. The temperature range in which a eutectoid steel (0.8% C) forms bainite is approximately 975-530oF (525 and 275oC). Since bainite shows a substantial variation in microstructure from the highest to the lowest temperatures of formation, the terms upper and lower bainite are used to more accurately described the microstructure.
 |
Upper bainite is rather featherly-appearing microstructure, while lower bainite is much more acicular (figure 7), resembling its close cousin, tempered martensite. Since bainite structures are composed of iron carbide and ferrite, often supersaturated with carbon, the distinction between upper and lower bainite is significant considering there can be major differences in mechanical properties. For the most part, bainite is harder, stronger and tougher at low temperatures than ferrite-pearlite or stright pearlitic microstructures, in steels of equivalent carbon contents. This microstructural interpretation becomes important when attempting to resolve failure mechanisms involving these steels in H2S gas (sour) environments. Unfortunately however, it can be extremely difficult to distinguish a steel microstructure as upper or lower bainite, and even at times with martensite.
Bainite is not referenced in the Fe-Fe3C phase disgram since its production involves faster cooling rates than those allowed for in this phase diagram. To predict the formation of bainite upon cooling from austenite, other diagrams must be used, specifically, isothermal transformation (ITT) diagrams, sometimes called time-temperature transformation (TTT) diagrams. These diagrams involve isothermal cooling, meaning cooling at a c
 |
onstant (iso) temperature (thermal).
Bain and his associates created many ITT diagrams for steel, though they have limited direct use in industrial applications since isothermal cooling conditions are rarely used outside the laboratory. However, modified ITT diagrams to accommodate continuous cooling conditions are useful for commercial practice. The most functional diagrams of this type are the modified continuous cooling transformation (CCT) diagram for engineering steels,of which a popular series was produced under the direction of M. Atkins of the British Steel Corporation.
[ Go to Top ]
Figure 8 shows the modified CCT diagram for a low carbon (0.18% C) steel. The diagram is read by drawing a vertical line from the section thickness (bar diameter) and cooling medium of interest, upwards to the top of the diagram. Following this line downwards from the A (austenite) region results in the room temperature microstructure produced upon continuous cooling within the selected medium. This information provides very useful data since microstructure prediction for industrial cooling is now possible and thus, properly prediction for the steel.
If austenite is very rapidly cooled, diffusion ocntrolled transformation to ferrite, pearlite and even bainite may not be possible. Instead, the austenite changes its crystal structure by a diffusionless shearing mechanism that moves blocks of a
 |
toms. The carbon originally dissolved in the solid solution of austenite,is now trapped in a ferrite structure.
Since ferrite has an extremely low solubility of carbon, its crystal structure becomes distorted to accommodate the presence of the trapped carbon, resulting in a volume expansion. This new microstructure is called martensite, named by Osmond in a tribute to Professor Adolf Martens, a German railway engineer who in 1878 started a center for metallographic research.
Martensite is no longer a true body-centered cubic phase, but rather a body-centered tetragonal (bct) structure (figure 9). The extreme distortion imposed by the carbon atoms is said to account for the substantially higher hardness and strength of this microstructure. The temperature at which austenite starts to transform to martensite is termed the Ms temperature a
 |
nd the temperature at which it is finished is called the Mf temperature. The maximum rate of cooling required to produce 100% martensite is called the critical cooling rate.
The atomic proof of carbon's effect on distorting, and thereby hardening the bct structure, is exhibited in figure 10, where increasing carbon content also increases the height or C dimension of the bct structure.
One would expect that steels of higher carbon content, being more distorted, would produce martensite of greater hardness, and this is in fact so, as figure 11 demonstrates. Consequently, not all martensitic structures are created equal, with their hardness, tensile strength, wear resistance and other mechanical properties controlled by the steel's carbon content. [ Go to Top ]
Martensite is the product of cooling austenite at a rate equal to or faster than the critical coo
 |
ling rate (figure 12). In order to produce martensite, one has to initially start with austenite, making austenite the mother of martensite. Figure 13 shows that martensite formation often initiates at the prior austenitic grain boundaries.
Martensite starts to form on rapid cooling at the Ms temperature. The Ms temperature decreases sharply with increasing carbon content in steels. All other alloying elements, such as Mn, Ni, Cr, Mo, lower the Ms, except for Co which raises the Ms. A significant effect of low Ms temperature is incomplete austenite to martensite transformation at room temperature. Therefore, as-quenched martensitic structure may retain austenite as partnof its room temperature microstructure. If left untransformed, the retain austenite at room temperature becomes an accident waiting to happen.
Although martensite can be a very hard, wear resistant, strong material, it lacks ductility, toughness and in all but low-carbon steels it is extremely brittle. Consequently, martensite must be heat treated to enable parts to be used for industrial purposes. Heat treatment reduces the internal strain in the bct structure, thereby increasing ductility and toughness, at some expense to hardness, wear resistance and strength.
A steel through-hardened to a martensitic structure is not a satisfactory engineering material for most applications. Despite its potential strength, it lacks ductility and toughness, often to the point where its full strength cannot even be measured since failure is so easily initiated. In order to develop ductility and toughness, the quenched steel is further treated by tempering
  |
Martensite is not a stable constituent, and on heating it will decompose to its stable products, ferrite and cementite. The extent of this decomposition will depend upon tempering temperature and time at temperature. At high tempering temperature and/or long periods of time, decomposition of martensite can be so complete that it approaches the mechanical properties of ferrite (soft, ductile,low strength and hardness). At low tempering temperature and/or short tempering times, decomposition is minimal and the nartensite remains hard and strong with slight increases in ductility and toughness. Thus, the appropriate choice of tempering temperature and time at temperature is required to achieve the specified mechanical properties necessary for the intended application.
In tempering fully quenched (martensitic) steels, it should be cautioned that a loss in ductility may result from prolonged heating between 500 and 650 oF (260 and 340 oC). Between these temperatures, the notch ductility of the steel (assessed by impact tests) is reduced. This phenomenon is called temper embrittlement or blue brittleness. [ Go to Top ]
The effect of all alloying elements is to reduce the rate at which martensite will temper. Thus, at a given tempering temperature, and for a given time, the alloy steel will show a greater hardness than the unalloyed steel. The design of steels and cooling conditions to produce required amounts of martensite are the subject of technology referred to as hardenability.
The measure of a steel's ability to harden to depth is its hardenability. Steels with high hardenability are those that require slower cooling rates for martensite formation. However, it is the carbon content of a steel that determines the maximum hardness feasible. The effect of carbon on hardness is demonstrated in figure 14
 |
An important factor influencing the maximum hardness that can be achieved is mass of the metal. In a small section, the heat is removed quickly, thus exceeding the critical cooling rate of the steel. As section size increases, it becomes increasingly difficult to remove the heat fast enough to exceed the critical cooling rate and thus avoid formation of nonmartensitic products.
An example of the mass effect is shown in figure 15, which illustrates the effect of section size on surface hardness. For small sections up to 0.5 inches (13 mm), full hardness of about 63 HRC is achieved. As the diameter of the quenched piece is increased, cooling rates and hardness decrease because the critical cooling rate for this specific steel was not exceeded. Thus, figure 15 also serves as an example of a low-hardenability steel. Plain carbon steels are characterized by their low hardenability, with critical cooling rates exceeded only in thin sections. Hardenability of all steels is directly related to critical cooling rates. The lower the critical cooling rate, the higher the hardenability for a given steel, almost regardless of carbon contant.
 |
Alloying steel with elements such as nickel, chromium, and molybdenum can also be used to make it more difficult for the diffusion controlled transformation of austenite to occur. As a result, martensite can be formed with less drastic cooling, such as oil quenching. Still greater alloying can yield "air hardenable" alloys. Slower cooling rates to produce martensite are beneficial since fast cooling introduces high surface residual stresses which may cause quench cracking. Quench cracks arise when a steel is quenched and undergoes stresses resulting both from thermal contraction and from a volume expansion (2 to 4%) which accompanies the transformation of austenite to martensite.
Although alloying elements can increase a steel's hardenability, they do not increase the steel's maximum hardness possible. Hardness is determined principally by the amount of carbon.
The factors which increase hardenability work not only to produce martensite but also to form other microstructures. Thus, hardness graduants in bars of various diameters, cooled at various rates, can be estimated. Continuous cooling transformation diagrams, such as in figure 8, demonstrate the various cooling conditions and related microstructures.
Metals generally consist of regions called crystals or grains where the atoms are arranged in regular geometric patterns such as bcc or fcc. Although the geometric pattern of atoms is fixed for grains of a particular material, the grains are oriented randomly with respect to the x, y, and z directions. As a result there is a disarray of atoms where the grains meet each other, called grain boundaries. This disarray of atoms along grain boundaries can be exposed by etching techniques that allow grains of the metal to be examined and measured.
Metallographic Examination [ Go to Top ]
Etching techniques are used on polished surfaces to reveal the metal grains and the various phases of the metal. Microscopic observation of this type is called metallographic examination and the metal images observed are called microstructures. Metal samples must be specificaaly
 |
prepared for the purpose and the science of sample preparation, examination and photography of the microstructures is called metallography.
To examine the microstructure of a metal with an optical microscope, the area to be examined is first polished. Polishing leaves a mirror-like metal surface which is smooth and highly reflecting, but covered with thin film of metal which is plastically deformed by the abrasive action of the final polishing operation (figure 16). To reveal the true metal structure, the deformed surface layer must be removed. The various structural components of the underlying metal can then be revealed. This is done by etching. Various etchants are used to best reveal the metal structure, but in general the etchants dissolve the distorted surface layer and then attack and dissolve the underlying metal. Metallographic etchants are very selective. Crystals of varying orientation are attacked more rapidly than the body of grains, and various structural components are attacked at different rates. Thus, by developing hills and valleys, plateaus of varying levels, etch pits of varying orientation, and similar differentiating effects, the structure of metal can be revealed.
In an optical microscope where light is passed through the microscope tube and reflected from the specimen to the observer's eye, the specimen appears bright. Where the intensity of reflected light is decreased by scattering from a roughened surface, the specimen appears less bright, and where the light is reflected so that none passes back through the microscope tube, the specimen appears dark (figure 16). Examples of ferrous microstructures are shown in figure 17.
The grain growth characteristics of hypoeutectoid steels taht have been deoxidized with silicon are said to be normal in that the austenitic grain size increases continuously and progressively as the austenitizing temperature is raised above the A3 temperature. Austenitic grain growth is also time dependent, the grains continuing to grow at any one temperature.
The austenitic grain size of annealed or normalized medium carbon steels can readily be observed because proeutectoid ferrite precipitates along the austenitic grain boundaries during slow cooling. Thus bands of ferrite outline pre-existing austenite grain boundaries
It is not so easy, however, to recognize the sites of the austenitic grain boundaries in low carbon steels when a large volume fraction of ferrite is present. Similarly, for quenched and tempered steels (martensitic), special etching techniques are required to reveal the prior austenitic grain size. A suggested etchant to reveal prior austenitic grains in steels fully hardened to martensite is 1 g of acid, 5 ml. of HCl and 95 ml. of ethyl alcohol (see ASTM Standard E112 appendix 3 for more details).
Grain coarsening of austenite is reversible. Several new grains of austenite can be nucleated in the volume that had been occupied by one former austenite grain, and that the size to which these new grains grows depends primarily on the new austenitizing temperature. Thus the new austenite grain size will generally be smaller than the former grain size if the new austenitizing temperature is lower than the previous one. [ Go to Top ]
 |
However, a small austenitic grain size is usually not always recovered in a single reaustenitizing heat treatment, depending on the initial size of the grains. For larger grain sizes, several reaustenitizing heat treatments may be required to obtain uniform and small final grain size. Keeping in mind that the lower the austenitizing temperature the greater the grain refinement.
Some steels are treated during the steelmaking process with grain refinement alloying elements, such as Al, Nb, V, Ti and Zr, which inhibits austenitic grain growth. The austenitic grain size after heating at normal austenitizing temperatures is then much smaller than for normal steels. The product then is commonly called fine-grained steel.
Reducing the ferrite grain size by this or other methods results in increased yield strength, which varies approximately with the reciprocal of the square root of the ferrite grain diameter (d-1/2). Reducing the ferrite grain size also increases the toughness, which is the one factor that improves both the yield strength and toughness simultaneously. For example, many proprieary line pipe steel specifications contain requirement on ferrite grain size to minimize the risk of brittle fracture.
Grain size is commonly measured according to ASTM Standard Method E112. Determining The Average Grain Size. This standard lists three methods for determining granin size, namely the comparison Procedure, Planimetric (Jeffrys') Procedure, and Intercept Procedure. Because of their purely geometric basis they are quite independent of the metal concerned and may also be used for the measurement of grain, crystal,or cell size of nonmetallic materials.
In materials having two or more constituents, the grain size usually refers to that of the matrix, except that in those materials where a second phase of sufficient amount, size or continuity to be significant, the grain size may be reported separately. Minor constituent phases, inclusions, and additives are not normally considered.
It is important in using these methods to recognize that the measurement of grain size is not precise, but an estimate. A metal grain is a three-dimensional shape of varying sizes. The grain cross section produced by random plane (surface of observation) is dependent upon where the plane cuts each individual grain. Thus, no two fields of observation can be exactly equal.
The comparison procedure is very popular since it takes the least time to carry out. This method involves viewing grains in a microscope and comparing them at the same magnification, 75X or 100X, to charts defined in ASTM E112, with two examples shown in figure 18. The ASTM Grain Size Number corresponds to a certain number of grain/in2 according to Table 1.
|
Table 1 |
|||||||||||
| ASTM No. | |||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Grains/in2 | |||||||||||
| 0.1 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | |
The relationship between the Grain Size Number and the number of Grains/in2 is given by the expression:
 |
n = 2 (N-1)
where N = ASTM Grain Size Number
n = number of Grains/in2 at the specified magnification.
In the planimetric (Jeffry's) procedure a known area is inscribed in the observed field and the grains within this area are counted and multiplied by the Jefferies' multiplier. The product will be the number of grains per square millimeter.
The intercept method has two procedures: the lineal (Heyn) procedure and circular procedure. Both methods involve placing a grid pattern on the field of observation and counting the number of grains at each intercept within a selected area.
Effect of Alloying Elements in Steel [ Go to Top ]
With alloying elements, it is important to determine whether they are carbide, austenite or ferrite formers and the purpose for being added to the steel. Each individual element transfers specific properties to the steel, according to the amount added. The presence of several elements can enhance one another, resulting in a synergistic effect. However, there are alloying elements that do not influence a particular property in the same direction as others and may in fact counteract one another. Alloying elements in steel only provides the potential for specific properties. These properties may not actually be achieved until processing and heat treatment have been carried out. The principal effects of the alloying elements on steel are as follows.
Carbon (C) Melting point 6404oF (3540oC)
C is the most important and influential alloying element in steel. In addition to carbon, however, any unalloyed steel will contain silicon,manganese, phosphorus and sulphur, which occur unintentionally during manufacture. The addition of further alloying elements to achieve special effects and intentional increase in the manganese and silicon contents result in alloy steel. With increasing C content, the strength and hardenability of the steel increase, but its ductility, forgeability, weldability and machinability (using cutting machine tools) are reduced. Corrosion resistance to water, acids,and hot gases is practically unaffected by the carbon.
Manganese (Mn) Melting point 2230oF (1221oC)
Mn is normally present in all commercial steels. It is essential to steel production, not only in melting but in rolling and other processing operations.
Mn deoxidizes steel. It compounds with sulphur to form Mn Sulphide (MnS), thus reducing the undesirable effect of the iron sulphide (FeS). This is of particular importance in free-cutting steel since it reduces the risk of hot shortness.
Mn reduces the critical cooling rate, thus increasing hardenability. Yield point and strength are increased by addition of Mn and, in addition, increases hardness penetration depth. Steels with Mn contents > 12% are austenitic because Mn is an austenite former and stabilizer. An example of 12% Mn steel is the Hadfield manganese steels that can achieve high degrees of work hardening, where the surface is subjected to impact stress while the core remains tough. For this reason, these high Mn steels are used in the mining industry in jaw crushers.
Steels with Mn contents of 18% or less remain unmagnetizable even after pronounced cold forming, as well as remaining tough at subzero temperatures. The coefficient of thermal expansion increases as a result of Mn, while thermal and electrical conductivity are reduced.
Silicon (Si) Melting point 2577oF (1414oC)
Si is contained in all steel in the same way as manganese, as iron ores incorporate a quantity of it according to their composition. Si is not a carbide former but enters into solution in the ferrite. Si is not a metal but a metalloid, as are phosphorus and sulphur.
One of the most important applications of silicon is its use as a deoxidizer in molten steel. Silicon is usually present in fully deoxidized alloy steels in the amounts up to 0.35%, insuring the production of sound, dense ingots. It promotes graphite precipitation and restricts the gamma phase significantly, increases strength and wear resistance (Si-Mn heat treatable steels), and significantly increases the elastic limit, thus being a useful alloying element in spring steels.
Aluminum (Al) Melting point 1216oF (658oC)
Al is used for deoxidation and for control of inherent grain size. When added to steel in small amounts, it produces a fine austenitic grain size. In fact, of all the alloy elements, aluminum in prescribed amounts, is the most effective in controlling grain growth. Titanium, zirconium, and vadanium are also effective grain qrowth inhibitors, but have adverse effects on hardenability because their carbide compounds are very stable and difficult to dissolve in austenite prior to quenching. Al does not form a acrbide.
Al is used as alloying addition in amounts of 0.95% to 1.30% in the most popular nitriding steel. The extremely high hardness of the nitrided case is due to the formation of a hard, stable aluminum nitride compound. The amount of Al present in nitriding steels is considerably in excess of the amount necessary to produce a fine austenitic grain size in other steels.
Phosphorus (P) Melting point 111oF (44oC)
P is usually regarded as a tramp element in steel since it is present in iron orc. P produces primary segregation on solidification of the steel melt and the possibility of secondary segregation in solid state due to the noticeable restriction of the gamma phase. It is difficult to achieve homogeneous distribution of P in steel, such that P contents are usually limited to 0.03-0.05%.
[ Go to Top ]
Sulphur (S) Melting point 244oF (118oC)
S produces the most pronounced segregation of all steel accompanying elements. Iron sulphide (FeS) leads to hot shortness, as the low melting point sulphide eutectics surround the grains, so that only slight cohesion between grains occur and during hot forming the grain boundaries tend to fracture. FeS also become susceptible to hydrogen-induced cracking in many environments, most notably where H2S gas is present, such that 0.001% S pipeline steels are common. As sulphur possesses a considerable affinity for manganese, it is combined in the form of Mn Sulphide (MnS) as this is the least dangerous of all inclusions, as it is distributed in point form in the steel. S significantly reduces toughness. It is added intentionally to steels for automatic machining up to 0.4%, as the friction on the tool cutting edge, reduced by sulphur's lubricating action, permits increased tool life. In addition, short chips occur when free-machining steels are machined. S decreases weldability by promoting hot cracking.
Chromium (Cr) Melting point 3488oF (1920oC)
Cr is a strong carbide and ferrite former that among several advantages, increases the edge-holding quality and wear resistance of steel cutting tools. Cr reduces the critical rate of cooling necessary for martensite formation, thereby increasing hardenability and allowing these steels to become oil and air-hardened. Notch toughness is reduced, but ductility suffers only slightly. Weldability decreases in pure chromium steels with increasing Cr content. The tensile strength of the steel increases by 11.5-14.5 ksi (80-100 N/mm2) per 1% Cr addition. While increasing Cr contents improve oxidation resistance, particularly at higher temperatures, a minimum content of about 12% chromium is necessary for corrosion resistance of steels, i.e. stainless steels.
Cr raises the A1 and A3 critical points, especially when large amounts of chromium are present. The eutectoid carbon content is found to be lowered by chromium additions, by an amount varying with quantity present. At 2.0% chromium, the eutectoid forms with 0.62% carbon. With 12.0% chromium, eutectoid carbon drops to under 0.40%.
Nickel (Ni) Melting point 2647oF (1453oC)
Nickel as an alloying element in alloy steels is an austenite former and is soluble in all proportions in both gamma and alpha iron. It is not a carbide former. In combination with chromium, nickel produces alloy steels with greater hardenability, higher impact strength, and fatigue resistance than possible with carbon steels. Ni produces a significant increase in notch toughness, even in the low temperature range, and is therefore alloyed for increasing toughness in case-hardening, heat-treatable and low temperature toughness steels.
Ni depresses the Ac and Ar critical points. It lowers the carbon content of the eutectoid which, with a 3.5% nickel steel, is reduced to 0.70% carbon.
As a result of increasing the gamma loop, Ni in content of > 7% imparts austenitic structure to stainless steels, down to well below room temperature. Ni on its own only makes the steel rust resistant, even in high percentages, but in austenitic Cr-Ni stainless steels (AISI 300 series), results in resistance to the effect of reducing chemicals. Resistance of these steels in oxidizing substances is achieved by means of Cr. At temperatures above 1100oF (593oC), austenitic steels have greater high temperature strength, as their crystallization temperature is high.
Molybdenum (Mo) Melting point 4752oF (2622oC)
Mo in steel can form a solid solution with the ferrite phase and also, depending on the Mo and carbon content, can form a complex carbide. Mo is ususlly alloyed together with other elements, such as Cr and Ni. Mo raises the Ac3 critical point when added in the usual amounts (0.10 to 0.60%) for alloy steels. When Mo is in solid solution in austenite prior to quenching, the reaction rates for the transformation of austenite become considerably slower as compared with a carbon steel, resulting in deeper hardening steel. A strong carbide former, the cutting properties with high speed steel are improved by Mo.
Mo steels in the quenched condition require a higher tempering temperature to attain the same degree of softness as comparable carbon or alloy steels. This resistance to tempering contributes to the ability of these steels to retain their strength at elevated temperatures. They show, because of this effect, a considerable resistance to "creep" under sustained loads below their elastic limit at temperatures up to 1100oF (593oC).
Mo promotes grain refinement and increases yield strength. It belongs to the elements which increase corrosion pitting resistance and is therefore used frequently with high alloy Cr steels and with austenitic CrNi steels. High Mo contents reduce susceptability to pitting corrosion,as in type 317 stainless steel containing 3.0-4.0% Mo.
Vanadium (V) Melting point 3139oF (1726oC)
V is a strong carbide former and promotes grain refinement. The complex carbides formed by V additions are quite stable, thus providing increase in wear resistance, edge holding quality and high temperature strength. Similarly, V offers significant improvement in retention of temper and reduction of overheating sensitivity are achieved with its addition. It is used primarily as additional alloying element in high speed, hot forming and creep resistant steels.
[ Go to Top ]
It dissolves to some degree in ferrite, imparting strength and toughness. As with other strong carbide formers, vanadium raises the critical points and decreases the carbon content of the eutectoid. V restricts the gamma phase and shifts the Curie point at elevated temperatures.
Titanium (Ti) Melting point 3141oF (1727oC)
On account of its very strong affinity for oxygen, nitrogen, sulphur and carbon, Ti has a promounced deoxidizing, dentriding, sulphur bonding and notable carbide forming action. Its carbide-forming tendency is so strong that a 0.50% carbon steel will have practically no tendency to quench harden when 1.5 to 2.0% Ti is addeed.
Used widely in stainless steels as carbide former for stabilization against intercrystalline corrosion, Ti also possesses grain refining properties. Ti is a strong ferrite former and stabilizer, thereby restricting the gamma phase. In high concentration, it leads to precipitation processes and is added to permanent magnet alloys on account of achieving high coercive force. Ti increases creep rupture strength through formation of nitrides.
Niobium/Columbium (Nb/Cb) Melting point 3542oF (1950oC) Tantalum (Ta) Melting point 5486oF (3030oC)
These elements occur almost exclusively together and are very difficult to separate to separate from one another, so that they are usually used together. They are very strong carbide formers, thus they are alloyed particularly as stabilizers of stainless steels. Both elements are ferrite formers and thus reduce the gamma phase. The A3 temperature is raised and the A4, or upper austenite limit, is lowered. Due to the increase in high temperature strength and creep rupture strength of Nb (Cb), it is frequently alloyed to high-temperature austenitic boiler steels. Ta has a neutron high absorption cross-section; only Ta/Nb (Cb) is considered for use in reactor steels.
One of the advantages of using Nb (Cb) for grain refinement is its low deoxidizing power does not introduce undesirable oxide inclusions into the steel. This fine grain size and the decreased hardenability attributed to columbium increases ductility of steels marginally and toughness significantly.
Boron (B) Melting point 4172oF (2300oC)
B is usually added to steel to improve hardenability, that is, to increase the depth of hardening during quenching and thus causes an increase in core strength in case-hardening steels. B-treated steels will usually have a B content in the range of 0.0005 to 0.003%.
Because B possesses a high cross section for neutron absorption, it is used to alloy steels for controllers and shields of atomic energy plants. A reduction in weldability must be expected in B alloyed steels.
Calcium (Ca) Melting point 1562oF (850oC)
Ca is used together with Si for deoxidation. It increases scaling resistance of heating conductor materials. It is also added to pipeline steels for use in sour (H2S) gas service to shape control (spherodize) nonmetallic inclusions, such as MnS.
Nitrogen (N) Melting point -346oF (-210oC)
As an alloying element, N extends the gamma phase and stabilizes the austenitic structure. In austenitic steels, such as the AISI 300 series of stainless steels, N increases strength and above all the yield point plus mechanical properties at elevated temperatures. As a result of nitride formation, N permits high surface hardness to be achieved during nitriding.
Selenium (Se) Melting point 423oF (217oC)
Se is used in free-machining steels to improve machinability. much like sulphur.
Crystal allotropes of iron, phases and microconstituents have been previously discussed and ithas been shown that steel can be thermally heated to a broad range of properties. Several heat treating terms are used to describe the thermal conditions under which different microstructures, phases, and crystals may be present. These are described as follows:
The annealing process is intended to optimize the steel's machinability and formability. In manufacturing steel products, machining and forming are often employed. Quenched and tempered steel may not machine or bend very easily and annealing is often necessary to manufacture steel components economically. Annealing is used after cold forming operations, since during forming, the deformed areas of the steel may become work-hardened and susceptible to fracture.
Steel is heated 50 to 100oF (10 to 38oC) above the A3 for hypoeutectoid steels, and above the Acm for hypereutectoid steels, with slow controlled cooling, resulting in soft andductile microstructures that have commercially maximized machinability and formability. In full annealing, cooling must take place very slowly so that a coarse pearlite is formed. When the term annealing is applied to steels it is assumed that full annealing was performed.
The process of normalizing consists of heating to a temperature 50 to 100oF (10 to 38oC) above the A3 and allowing the part to cool in still room temperature air. The actual temperature required for this depends on the composition of the steel, but is usually around 1550 to 1650oF (840 to 900oC) for most low and medium carbon steels. Normalizing can be described as a homogenizing or grain-refining treatment. Within any piece of steel, the composition is usually not uniform throughout. That is, one area may have more carbon than the area adjacent to it. These compositional differences affect the way in which the steel will respond to heat treatment. If the steel is austenitized, the carbon can readily diffuse throughout, and the result is a reasonably uniform composition from one area to the next. The steel is then more homogeneous and will respond to the heat treatment is a more uniform way.
The grain-refining effects of normalizing become of prime importance in designing steel microstructures for low temperature service. By refining the grain size, more grain boundaries are formed and the energy necessary to impact fracture is increased in order to "push" the crack across the grain boundaries. For example, ASTM A 516 grade 70 pressure vessel plate steel is commonly specified to have a notch toughness (Charpy) of 20 ft-lb (27 J) minimum at -50oF (-46oC). To achieve this notch toughness requirement, an ASTM grain size No. 7 or similar is typical and is most commonly attained by normalizing.
A fully hardened steel is defined as having a 100% martensitic structure, since it is the hardest strucutre that the steel can obtain. In order to achieve a complete martensitic structure, austenite must first be formed throughout the section thickness of the steel, called austenitizing. To ensure complete austenitization through-thickness of the part, the appropriate temperature above A3 must be reached for a sufficient amount of time. This is followed by rapid cooling (quenching) in water, oil or air, with or without agitation, depending on the hardenability of the particular steel.
Tempering is generally applied to hardened or quenched steel to improve mechanical properties, for the most part, tensile strength, ductility and toughness. Tempering, formerly called drawing, is performed by heating a quenched part to some point below the lower critical transformation temperature for sufficient time depending on its size, commonly 2 hours or more. Most steels are tempered between 400 to 1100oF (205 to 595oC). As higher temperatures or longer periods of time are employed, toughness and ductility are increased, but at the expense of reduced hardness and tensile strength. The microstructure of quenched and tempered steel is referred to as tempered martensite.
When a metal is heated, expansion occurs which is proportional to the temperature rise. Similarly, upon cooling a metal, contraction occurs. When a steel component is heated at one point more than at another, as in welding or during forging, internal stresses are set up. During heating, expansion of the heated area do not occur uniformly, and the component tends to distort. On cooling, contraction is restricted from occurring by the unyielding cold metal surrounding the heated area, as in a weldment. The forces attempting to contract the metal are not relieved, and when the metal is cold again, the forces remain as internal stresses, also called residual stresses. Stresses also result from volume changes which accompany phase transformations, such as the transformation of austenite to martensite.
Residual stresses are harmful because they may cause distortion of steel parts and/or may render the part susceptible to brittle fracture and stress corrosion cracking mechanisms. To relieve these stresses, plain carbon steel is typically heated to between9400 to 1100oF (482 to 595oC), assuring that the entire part is heated uniformly, then slowly cooled back to room temperature. This procedure is called relief annealing or, more commonly, stress relieving.
AN INTRODUCTION TO THE
WELDING METALLURGY OF STEEL
Welding is the joining of two or more pieces of metal by applying heat or pressure, or both, with or without the addition of filler metal, to produce a localized union through fusion or recrystallization across the interface.
[ Go to Top ]
In industrial welding practice, a steel of one composition, such as pipeline or pressure vessel steel, is likely to be welded with a steel electrode of different chemical composition, such as AWS A5.1 classification E7018. The majority of filler metals classified by AWS, CSA, and other standards are based on providing crack-free welds and mechanical properties that at least meet the minimum requirements of the base metal. The chemical composition match, although important, is a secondary consideration for carbon steels. However, matching filler metal chemistry to base metal chemistry becomes increasingly important when welding alloy and stainless steels.
As a result of the chemically nonmatching filler metal, heat distribution, and electrical arc characteristics, the weld joint is usually a chemically heterogeneous composite consisting of as many as six metallurgical distinct regions.
A typical single pass weld is shown in figure 19 and consists of:
| 1. Composite zone | 4. Partially melted zone | |
| 2. Unmixed zone | 5. Heat-affected zone | |
| 3. Weld interface | 6. Unaffected Base metal |
Composite Zone
The combination of melted filler metal and melted base metal creates a liquid weld pool that becomes the composite zone upon cooling. Should the filler metal be of a different chemical composition compared to the base metal, then the base metal is said to become diluted by the filler metal. However, due to the electrical sitrring action of the welding arc and thermodynamic forces, the composite zone is mainly homogeneous.
 |
Unmixed Zone
A very tin region, typically 0.05 - 0.01 in (1.25 - 2.5 mm) surrounding the composite zone is called the unmixed zone. The metal in this region solidified prior to mixing with the filler metal since the temperature reached was just above its melting point. The stirring action and time above the melting temperature is insufficient for mixing to have taken place. Therefore its chemical composition is essentially the same as the base metal. Although the unmixed zone is present in all fusion welds, it is readily visible only in those welds using a filler metal alloy of substantially different chemical composition than the base metal.
The next zone metallurgically defined in a weldment is the weld interface, typically called the weld line or fusion line. This interface clearly separates the unmelted base metal on one side and the solidified weld metal on the other side. In pure metals, the transition from base metal to weld metal is often difficult to observe metallographically because of epiaxial qrowth, where during liquid weld metal solidification, the new solid crystals begin to grow from the existing base metal grains. However, in carbon and alloy steels, the weld interface is easily revealed by standard etching techniques. This method is often used in the "field"to delineate the weld metal (copmosite and unmixed zones) from the heat affected zone in carbon steel welds. Two common macroetchants for this purpose are: 5 to 10% Nital (5 to 10% concentrated nitric acid dissolved in 90% to 95% methanol or ethyl alcohol, by volume) and 10% Ammonium Persulphate (10 g ammonium persulphate dissolved in 100 mL of water).
Partially Melted Zone
In the base metal immediately adjacent to the weld interface, the temperature from welding is insufficient for complete melting, i.e. just below the liquidus temperature but above the solidus temperature, in the solid-liquid region. This area is called the partially melted zone. In steels, this zone becomes susceptible to hot cracking since the liquidation (melting) of manganese sulphide inclusions can cause weak localized regions that do not have the hot strength to withstand the heating/cooling (expansion/contraction) cycle of welding.
The HAZ is the subject of continuing interest since it involves a wide range of temperatures from the welding operation that can significantly alter the base metal's metallurgy and associated mechanical and physical properties. See the next section.
Unaffected Base Metal [ Go to Top ]
The further region from the liquid weld metal (composite zone) is the unaffected base metal. This region is defined by welding temperature below the lower critical transformation temperature (A3), 1340oF (725oC) for carbon steels. As such, there are no phase transformations taht occur to the original base metal microstructure. However, since the temperature reached in this region are sufficient to cause precipitation hardening, tempering or stress relieving, it is important to know the original microstructure and the potential effects of further heating.
HEAT AFFECTED ZONE (HAZ)
Single-Pass Weldments
The heat affetced zone (HAZ) extends from weld interface to the unaffected base metal. The resultant temperature range in the HAZ extends from just below the liquidus down to the sub-critical temperatures slightly less than the lower critical transformation temperature, i.e. 2970 - 8
 |
00oF (1350 - 425oC) for carbon steel. With this large temperature gradient comes varying microstructures in steel that will depend on the peak temperature reached, time at temperature, and cooling rate. Consequently, the term "heat affected zone" is really a misnormer when describing it on a metallurgical basis, since the HAZ is really made up of several distinct metallurgical zones.
Figure 20 shows a cross section of a single-pass weldment outlining the weld metal and HAZ. Because of varying thermal conditions as a function of distance from the weld interface, the HAZ is actually composed of four distinct regions, namely, the grain-coarsened-HAZ, grain-refined-HAZ, intercritical_HAZ, and subcritical-HAZ. Each of these regions within the HAZ possesses microstructures and associated mechanical and physical properties that make them unique.
To define the four regions of the HAZ in metallurgical terms, the Fe-Fe3C phase diagram provides an ideal tool. Figure 21 illustrates a single-pass weldment and compares the HAZ microstructures produced by the heat of welding and related to peak temperature reached, time at temperature, and cooling rate.
 |
The peak temperatures reached in the grain-coarsened-HAZ region raange from 2000 to 2700oF (1090 to 1480oC), depending on the carbon content. Another way to describe this temperature range in metallurgical terms, is that it exrends from much above the upper critical transformation temperature to just below the solidus temperature. There are two main metallurgical conditions that occur in this region: 1) the microstructure is austenite (for the most part) and 2) since the austenite produced is much above the upper critical transformation temperature, grain growth will occur. The amount of grain growth will depend on the peak temperature and time at that temperature, i.e. the higher the peak temperature and the longer the time at that temperature, the larger the austenite grains will grow.
Two significant metallurgical consequences result in this region: 1) since austenite is produced, the potential for transformation to martensite upon cooling exists, where martensite is not a desirable transformation product due to its lack of ductility, toughness, and susceptibility to cold cracking and 2) as austenitic grain size grows, the resultant room temperature microstructure will be similarly efr
effected, with low temperature notch (charpy) toughness being significantly change, i.e. the larger the grain size, the lower the notch toughness.
Grain-refinement-HAZ [ Go to Top ]
This region comprises temperature from just above the lower critical transformation temperature and up to 200oF (93oC) higher. These temperatures are within the normalizing heat treatment range and are very conducive to austenitic grain refinement and its associated improved low temperature notch (charpy) toughness. On the other hand, austenite is still produced and the likelihood of martensite must be considered.
The temperatures in this region includes the intercritical ranges, between the lower and upper critical temperatures. Some austenite is produced in this partially transformed range, such that the potential for martensite transformation exists. In medium and high carbon steels, this austenite can contain large amounts of carbon which has a higher tendency to produce martensite on cooling.
The subcritical-HAZ includes the tempered area of the Fe-Fe3C phase diagram. Should the base metal be in the tempered condition (i.e. quenched and tempered) the heat of welding may be sufficient for further tempering, thereby reducing the tensile strength and hardness in this region. There are no phase transformations which take place in the tempered area since the lower critical transformation temperature is not exceeded.
Cooling Rate
The cooling rate also varies from region to region in the HAZ. It increases with increasing peak temperature ay constant heat input and decreases with increasing heat input at constant peak temperature. Figure 21 displays a typical cooling rate curve (solid line showing peak temperature) for a single-pass weldment. By drawing a tangent to this curve, it becomes apparent that the steepest part of the curve (i.e. fastest cooling rate) is related to the grain-coarsened-HAZ. Since this region is comprised essentially of austenite and is linked to the fastest cooling r
 |
ate, it therefore possesses the greatest potential for transformation to martensite.
Toe cracking is a form of hydrogen-assisted cold cracking related to welding and owes its name to the area of the weld where cracking initiates. The toe of the weld is in the extreme grain-coarsened-HAZ (adjacent to the weld interface) and is directly affiliated with the austenite to martensite transformation in this high cooling rate region; a consequence of welding that produces the highest potential hardness in the HAZ of a carbon steel (see figure 22).
In multi-pass weldments, the situation is much more complex because of the presence of reheated zones within the HAZ. The reheating of the HAZ microsturctures by subsequent weld passes increases the inhomegeneity of the various regions with respect to microstructure and mechanical properties. Reaustenitization and subcritical heating can have a significant effect on the subsequent structures and properties of the HAZ. The loss of low temperature notch (charpy) toughness in a multi-pass HAZ is related to small regions of limited ductility and low cleavage resistance within the grain-coarsened-HAZ that are known as the localized brittle zones. At an adjacent weld interface in the multi-pass HAZ, the localized brittle zones may become aligned. These aligned brittle zones offer short and easy paths for crack propagation. Consequently, fracture may occur along the fusion line.
Welding Metallurgy Summary
The following Chapters/Tables list some of the more practical metals data contained in today's engineering standards. Many of the various organizations that issue metal standards and specifications throughout the world are included. One prominent organization is the Americam Society for Testing and Materials (ASTM). The following is an excerpt from the 1991 Annual Book of ASTM Standard Volume 00.01, describing the identification for individual ASTM Standards:
Each ASTM Standard has a unique serial designation. It is comprised of acapital letter indicating general classification (A, ferrous metals; B, non-ferrous metals; C, cementitious, ceramic, concrete, and masonry materials; D, miscellaneous materials; E, miscellaneous subjects; F, material for specific applications; G, corrosion, deterioration, and degradation of materials; ES, emergency standards; P, proposals), a serial number (one to four digits), a dash, and the year of issue.
In each serial designation, the number following the dash indicates the year of original adoption or, in the case of revision, the year of last revision. Thus, standards adopted or revised during the year 1991 have as their final number, 91. A letter following this number indicates more than one revision during that year, that is 91a indicates the second revision in 1991, 91b the third revision, etc. Standards that have been reapproved without change are indicated by the year of last reapproval in parentheses as part of the designation number, for example, (1991). A superscript epsilon indicates an editorial change since the last revision or reapproval; e1 for the first change, e2 for the second change, etc.
If a standard is written in acceptable metric units and has a comparison standard written in inch-pound units (or other units), the metric standard is identified by a letter M after the serial number; this standard contains "hard metric" units.
If s standard is written in inch-pound units (or other units) and acceptable metric units, the document is identified by a dual alphanumeric designation.
When reference is made to a standard, the complete designation should be given. Best practice is to state the designation and title. The boldface number(s) following the title refer to the volume(s) of the Annual Book of ASTM Standards in which the standard appears.
[ Go to Top ]
