Basic Concepts of Chemical Bonding
Lewis Symbols and the Octet Rule
Why are some substances chemically bonded molecules and others are an association of ions?
- depends upon the electronic structures of the atoms
- nature of the chemical forces within the compounds
A broad classification of chemical forces:
- Ionic bonds
- Covalent bonds
- Metallic bonds
Ionic bonds - electrostatic forces that exist between ions of opposite charge
- typically involves a metal with a nonmetal
Covalent bonds - results from the sharing of electrons between two atoms
- typically involves one nonmetallic element with another
Metallic bonds
- found in solid metals (copper, iron, aluminum)
- each metal bonded to several neighboring groups
- bonding electrons free to move throughout the 3-dimensional structure
Lets look at the preferred arrangements of electrons in atoms when they form chemical compounds
Lewis Symbols and the Octet Rule
Valence electrons reside in the outer shell and are the electrons which are going to be involved in chemical interactions and bonding (valence comes from the Latin valere, "to be strong").
Electron-dot symbols (Lewis symbols):
- convenient representation of valence electrons
- allows
you to keep track of valence electrons during bond formation
- consists of the chemical symbol for the element plus a dot for each valence electron
Sulfur
Electron configuration is [Ne]3s23p4, thus there are six valence electrons. Its Lewis symbol would therefore be:

Note:
- The dots (representing electrons) are placed on the four sides of the atomic symbol (top, bottom, left, right)
- Each side can accommodate up to 2 electrons
- The number of valence electrons in the table below is the same as the column number of the element in the periodic table (for representative elements only)
Atoms often gain, lose, or
share electrons to achieve the same number of electrons as the noble gas
closest to them in the periodic table
Because all noble gasses (except He) have filled s and p valence orbitals (8 electrons), many atoms undergoing reactions also end up with 8 valence electrons. This observation has led to the Octet Rule:
Atoms tend to lose, gain, or
share electrons until they are surrounded by 8 valence electrons
Note: there are many exceptions to the octet rule (He and H, for example), but it provides a useful model for understanding the basis of chemical bonding.
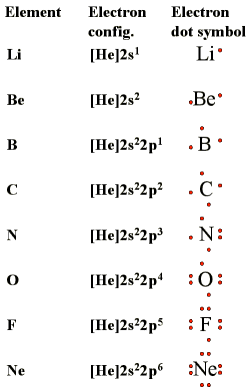
Ionic Bonding
Sodium metal reacts with chlorine gas in a violently exothermic reaction to produce NaCl (composed of Na+ and Cl- ions):
2Na(s) + Cl2(g) -> 2NaCl(s)
These ions are arranged in solid NaCl in a regular three-dimensional arrangement (or lattice):

The chlorine has a high affinity for electrons, and the sodium has a low ionization potential. Thus the chlorine gains an electron from the sodium atom. This can be represented using electron-dot symbols (here we will consider one chlorine atom, rather than Cl2):

The arrow indicates the transfer of the electron from sodium to chlorine to form the Na+ metal ion and the Cl- chloride ion. Each ion now has an octet of electrons in its valence shell:
Na+ 2s22p6
Cl-
3s23p6
Covalent Bonding
Ionic substances:
- usually brittle
- high melting point
- organized into an ordered lattice of atoms, which can be cleaved along a smooth line
the electrostatic forces
organize the ions of ionic substances into a rigid, organized three-dimensional
arrangement
The vast majority of chemical substances are not ionic in nature
- gases and liquids, in addition to solids
- low melting temperatures
G.N. Lewis
reasoned that an atom might attain a noble gas electron configuration by sharing electrons
A chemical bond formed by sharing
a pair of electrons is called a covalent bond
The diatomic hydrogen molecule (H2) is the simplest model of a covalent bond, and is represented in Lewis structures as:

The shared pair of electrons provides each hydrogen atom with two electrons in its valence shell (the 1s) orbital.
In a sense, it has the electron
configuration of the noble gas helium
When two chlorine atoms covalently bond to form Cl2, the following sharing of electrons occurs:

Each chlorine atom shared the bonding pair of electrons and achieves the electron configuration of the noble gas argon.
In Lewis structures the bonding pair of electrons is usually displayed as a line, and the unshared electrons as dots:

The shared electrons are not located in a fixed position between the nuclei. In the case of the H2 compound, the electron density is concentrated between the two nuclei:

The two atoms are bound into
the H2 molecule mainly due to the attraction of the positively
charged nuclei for the negatively charged electron cloud located between them
For the nonmetals (and the 's' block metals) the number of valence electrons is equal to the group number:
|
Element |
Group |
Valence electrons |
Bonds needed to form valence octet |
|
F |
7A |
7 |
1 |
|
O |
6A |
6 |
2 |
|
N |
5A |
5 |
3 |
|
C |
4A |
4 |
4 |
Examples of hydride compounds of the above elements (covalent bonds with hydrogen:

Thus, the Lewis bonds successfully describe the covalent interactions between various nonmetal elements
Multiple bonds
The sharing of a pair of electrons represents a single covalent bond,
usually just referred to as a single bond
In many molecules atoms attain
complete octets by sharing more than one pair of electrons between them.
Two electron pairs shared a double bond
Three electron pairs shared a triple bond

Because each nitrogen contains 5 valence electrons, they need to share 3 pairs to each achieve a valence octet.
- N2 is fairly inert, due to the strong triple bond between the two nitrogens
- The N - N bond distance in N2 is 1.10 Å (fairly short)
From a study of various Nitrogen containing compounds bond distance as a function of bond type can be summarized as follows:
- N-N 1.47Å
- N=N 1.24Å
- N=N 1.10Å
As a general rule, the distance
between bonded atoms decreases as the number of shared electron pairs increases
Drawing Lewis Structures
The general procedure...
1. Sum the valence electrons from all atoms
- Use the periodic table for reference
- Add an electron for each indicated negative charge, subtract an electron for each indicated positive charge
2. Write the symbols for the atoms to show which atoms are attached to which, and connect them with a single bond
- You may need some additional evidence to decide bonding interactions
- If a central atom has various groups bonded to it, it is usually listed first: CO32-, SF4
- Often atoms are written in the order of their connections: HCN
3. Complete the octets of the atoms bonded to the central atom (H only
has two)
4. Place any leftover electrons on the central atom (even if it
results in more than an octet)
5. If there are not enough electrons to give the central atom an
octet, try multiple bonds (use one or more of the unshared pairs of electrons
on the atoms bonded to the central atom to form double or triple bonds)
Draw the Lewis structure of phosphorous trichloride (PCl3)
This is an example of a central atom, P, surrounded by chlorine atoms
1. We will have 5(P) plus 21 (3*7, for Cl), or 26 total valence electrons
2. The general symbol, starting with only single bonds, would be:

3. Completing the octets of the Cl atoms bonded to the central P atom:

4. This gives us a total of (18 electrons) plus the 6 in the three single bonds, or 24 electrons total. Thus we have 2 extra valence electrons which are not accounted for. We will place them on the central element:

5. The central atom now has an octect, and there is no need to invoke any double or triple bonds to achieve an octet for the central atom. We are finished.
Draw the Lewis structure for the NO+ ion
1. We will have 5 (N) plus 6 (O) minus 1 (1+ ion), or 10 valence electrons
2. The general structure starting only with single bonds would be:

3. Completing the octet of the O bonded to N:

4. This gives us a total of 6 plus 2 for the single bond, or 8 electrons. There are 2 unaccounted for electrons and we will place them on the N:

5. There are only 4 atoms on the N atom, not enough for an octet, so lets try a double bond between the N and O:

The oxygen still has an octet, but the N only has 6 valence electrons, so lets try a triple bond:

Each atom now has a valence octet. We are finished.
The brackets with the + symbol
are used to indicate that this is an ion with a net charge of 1+
Formal Charge
In some cases we can draw several different Lewis structures which fulfill the octet rule for a compound. Which one is the most reasonable?
One method is to tabulate the valence electrons around each atom in a Lewis structure to determine the formal charge. The formal charge is the charge that an atom in a molecule would have if we considered each atom to have the same electronegativity in a compound.
To calculate formal charge, assign electrons to their respective atoms as follows:
- All of the unshared electrons are assigned to the atom on which they are found
- The bonding electrons are divided up equally between each atom involved in the bond
- The number of valence electrons expected in the isolated atom is compared to the actual number of electrons assigned from the Lewis structure:
The formal charge equals the
number of valence electrons in the isolated atom, minus the number of electrons
assigned in the Lewis structure
Example: Carbon Dioxide (CO2)
Carbon has 4 valence electrons
Each oxygen has 6 valence electrons, therefore our Lewis structure of CO2 will have 16 electrons:
![]()
One way we could draw the Lewis structure is:
![]()
Another way we could draw the Lewis structure is:
![]()
Both structures fulfill the octet rule. But what are the formal charges?


Which structure is correct? In general, when several Lewis structures can be drawn the most stable structure is the one in which:
- The formal charges are the smallest
- Any negative charge is found on the most electronegative atom
In the above case, the second structure is the one with the smallest formal charges (i.e. 0 on all the atoms).
- Furthermore, in the first possible Lewis structure the carbon has a formal charge of 0 and one of the oxygens it is bonded to has a formal charge of +1.
- Oxygen is more electronegative than Carbon, so this situation would seem unlikely.
It is important to remember that formal charges do not represent the actual charges on the atoms. Actual charges are determined by the electronegativity of the atoms involved.
1996 Michael Blaber