Worksheet 8.2, 8.3: Predicting the Products of
Replacement Reactions
SINGLE REPLACEMENT REACTIONS
A reaction in which one element takes the place
of another element as part of a compound, is called a single replacement
reaction. In this type of reaction, a metal always replaces
another metal and a nonmetal always replaces another nonmetal. The
general equation for a single replacement reaction is:
A + BC ® AC + B
Predicting if a reaction will occur:
Activity Series: Halogens:
There is an interesting regularity observed in replacement reactions involving the halogens: Fluorine, Chlorine, Bromine, and Iodine. Each halogen will react to replace any of the halogens below it in the periodic table, but will not replace those above. For example, chlorine will replace Bromine and Iodine, but it will not replace Fluorine.
Activity Series: Metals:
Metals also undergo replacement reactions, and regularities similar to those described for the halogens are observed. Metals can be listed in a series in which each metal will replace all the metals below it on the list, but none of the metals above it. Such list is commonly called an activity series. The activity series for metals is determined by experiments in which pairs of metals are compared for reactivity.
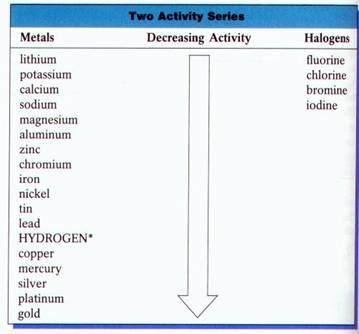
Use the
Activity Series to predict whether the following reaction can occur under
normal conditions.
Mg (s) +
CuSO4 (aq) ® Cu (s) + MgSO4 (aq)
Answer:
Since
magnesium is above copper on the activity series, magnesium is more active and
will replace copper in the compound CuSO4. This reaction will occur.
DOUBLE REPLACEMENT
REACTIONS
The general equation for a double replacement reaction is:
AB + XY ® AY + XB
Predicting Double Replacement Reactions:
Deciding whether a double replacement reaction
will occur is a matter of predicting whether an insoluble product can form.
If sodium nitrate is substituted for lead nitrate, you will see no reaction
when the solutions in the two test tubes are mixed. Why Not? If you write out
all possible combinations of metal and nonmetal ions and check the table below,
you will see that none of them is insoluble. The combinations of a number of different
positive and negative ions to form precipitates and soluble compounds.
Let’s take an example.
AgNO3 + KCl ® AgCl(s) + KNO3
Above is the way the reaction might be published
in a book, but the equation does not tell the whole story. Dissolved silver
nitrate becomes a solution of silver ions and nitrate ions. Potassium chloride
ionizes the same way. When the two solutions are added together, the silver
ions and chloride ions find each other and become a solid precipitate.
(They ‘rain’ or drop out of the solution, this time as a solid.) Since silver
chloride (See Chart on the next page) is insoluble in water, the ions
take each other out of the solution.
Ag+1 + (NO3)-1
+ K+1 +Cl-1 ® AgCl + K+1 + (NO3)-1

Here is another way to take the ions out of
solution. Hydrochloric acid and sodium hydroxide (acid and base) neutralize
each other to make water and a salt. The hydrogen and hydroxide ions take each
other out of the solution by making a covalent compound (water).
HCl
+ NaOH ® H2O
+ NaCl
Or
H+1 + Cl-1
+ Na+1 + (OH)-1 ®
HOH + Na+1 + Cl-1
One more way for the ions to be taken out of the water is for some of the
ions to escape as a gas.
CaCO3 + 2 HCl ® CaCl2 +
H2O + CO2
Ca+2 + (CO3)-2
+ 2 H+1 + 2 Cl-1 ®
Ca+2 + 2 Cl-1 + H2O + CO2
The carbonate and hydrogen ions became water and
carbon dioxide. The carbon dioxide is lost as a gas to the ionic solution, so
the equation can not go back.
One way to consider double replacement reactions is as follows: Two solutions of ionic compounds are really just sets of dissolved ions, each solution with a positive and a negative ion material. The two are added together, forming a mixture of four ions. If two of the ions can form (1) an insoluble material, (2) a covalent material such as water, or (2) a gas that can escape, it qualifies as a reaction.
Information taken from:
http://www.avon-chemistry.com/chem_intro_lecture.html