5.1: Satellite
a) Heat transported by radiation is important here.
b) J = σSB*T4
A = 4πr2
P = J*A = (σSB*T4)(4πr2)
c) T4 = P/(σSB*4πr2)
5.2: Heat Flow, Entropy and Free Energy
a) C = (ΔU/ΔT) = Q/ΔT
b) ΔS1 = C*ln(Tf/TCi)
c) ΔStot = C*ln(Tf/TCi) + C*ln(Tf/THi)
d) ΔF = ΔU - T*ΔS = -Tf*ΔStot
5.3: Calculating Entropy Changes
a) If the two halves contain the same gas (e.g., both are oxygen), the entropy will remain almost the same (ie, a reversible process).
b) If the two halves contain different gases (e.g., oxygen and nitrogen), the entropy will increase significantly.
c) ΔS = nR*ln(Vf/Vi) = (pV/T)*ln(Vf/Vi)
d) Q=α*nR*(Tf-Ti) :: solve for Tf
ΔS = Cv*ln(Tf/Ti) = α*nR*ln(Tf/Ti)
5.4: Adiabatic Processes
a) ΔSV = nR*ln(Vf/Vi) = (pV/T)*ln(Vf/Vi)
b) ViTia=VfTfa :: solve for Tf
c) ΔST = α*nR*ln(Tf/Ti) = α*(pV/T)*ln(Tf/Ti)
d) ΔStot = ΔSV + ΔST = 0
5.5: Soup and Free Energy
a) T = the room temperature
b) dU = Cv dT
dU = 26T dT ... Integrating this makes
Uextra = 13Tf2 - 13Ti2
c) dS = dU/T
dS = Cv/T dT
dS = 26T/T dT
dS = 26 dT ... Integrating this makes
Sextra = 26Tf - 26Ti
d) W = U - TiS
e) fraction = W/Uextra
NOTE ABOUT HOMEWORK B: THESE ANSWERS MAY NOT BE CORRECT. USE THEM AT YOUR OWN RISK.
HomeworkB 05
------------
The first two questions concern the heat engine shown:
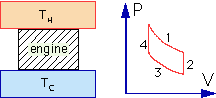 1) Over one complete cycle (1,2,3,4) of the engine, what happens to the entropy of the "working fluid" inside the engine?
1) Over one complete cycle (1,2,3,4) of the engine, what happens to the entropy of the "working fluid" inside the engine?
stays the same
2) Over one complete cycle of the engine, what happens to the entropy of the hot reservoir?
decreases (heat going out, entropy decreases)
2.Alternate) Over one complete cycle of the engine, what happens to the entropy of the cold reservoir?
increases (heat going in, entropy increases)
3) Now consider a stone with heat capacity C = 1.40 kJ/K which is left outside on a cold day to reach a temperature of 0°C. The stone is then brought inside where the air temperature is 20°C. The stone is used as the cold side of a Carnot engine. (The air is the hot side.) What is the maximum work that can be accomplished?
ΔU = CΔT
ΔS = C*ln(Tf/Ti)
ΔF = ΔU - TΔS
W = -ΔF = T*C*ln(Tf/Ti) - CΔT (use T=room temp)
In the next two questions, consider ideal gas particles in contact with a thermal reservoir at temperature T= 323 K. The probability of finding a particle in the energy range E to E+ΔE given by P(E)ΔE = CE1/2e-E/kTΔE.
4) If energy E1 is lower than energy E2, E1 < E2, which of the following statements must be true:
we do not have enough information to decide if P(E1) is greater or smaller than P(E2
Look at a boltzmann graph. At some places E1 is greater than E2, and vice versa.
5) Calculate the ratio of the probability that we find a particle with energy between E2 = 2kT and 2.1 kT to the probability that we find a particle with energy E1 = 1 kT and 1.1 kT . (Do not integrate, just multiply appropriate P(E) by the appropriate ΔE.)
[P(E2)*ΔE2] / [P(E1)*ΔE1] = [CE21/2e-E2/kT*ΔE2] / [CE11/2e-E1/kT*ΔE1]

