|
Links
Home
Sustainability
Nuclear contamination
Greenhouse effect
Ozone depletion
Toxic chemicals
Parco dei Colli
|

|
What is ozone ?
Ozone is a bluish, very reactive gas, whose molecule is made
by three oxygen atoms . (In the NASA image, one ozone
molecule is formed through the collision of an oxygen atom
with one biatomic oxygen molecule).
Nearly 90% of the Earth's ozone is situated in the
stratosphere , the atmosphere layer between 10 to 40 kilometers
above Earth's surface, where it is continuously generated and
destroyed by the UV radiation.
|
Only a minor part of ozone is in the troposphere, the internal
atmospheric layer, where the meteorological
phenomena occur. Tropospheric ozone is mainly produced by
photochemical reactions involving other
pollutant gases, specially over large cities.
|
 |
May ozone be dangerous ?
The thin layer ( NASA image) of ozone gas in the stratosphere
(ozone layer) is shielding life on earth from the harmful UV light
coming from the sun ( "good ozone").
Ozone is harmful at earth level , being very reactive and irritant
to the human eyes ( the so-called "bad ozone" ).
|
Is ozone layer threatened?
The overall amount of ozone is essentially stable in a
natural cycle. This has been true for millions of years .
Since some decades, according to atmospheric measurements, ozone layer is
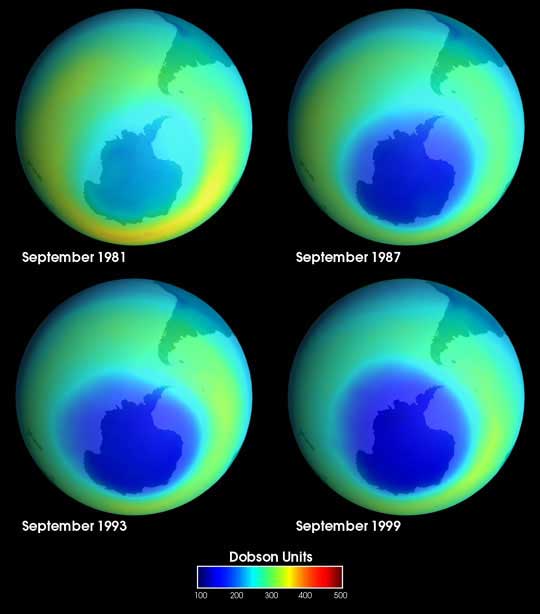
getting thinner. Ozone depletion has been most severe at the poles, specially over Antarctica, where a seasonal
ozone layer "hole" appears ( in the NASA image
the blue color means lack of ozone).
The Antarctic ozone hole was discovered in 1985 by British scientists :
it is not technically a “hole” where no ozone is present,
but is actually a region of exceptionally depleted ozone
in the stratosphere over the Antarctic that happens at the
beginning of Southern Hemisphere spring (August-October). An endlessly circling whirlpool of stratospheric winds called the "polar vortex" isolates the air over Anctartica in winter. The ozone hole grows throughout the early spring until temperatures warm and the polar vortex weakens, ending the isolation of the air. As air from the surrounding latitudes mixes into the polar region, the ozone layer stabilizes until the following spring.
|
 |
According to scientists, certain man-made chemicals are
major contributors to the problem. These chemicals are called
Ozone-Depleting Substancies ( ODS) and include many gases
containing chlorine and bromine, such as: chlorofluorocarbons
(CFCs, substances containing chlorine, fluorine and carbon) used
in refrigerators and blowing agents for foams; the
"Halons", used for fire fighting; methyl bromide, used
in agriculture.
Since the second world war, CFCs have been widely employed,
mainly because they are chemically inert and, as a consequence,
non toxic and extremely stable.
CFCs do not dissolve in rain : after several years, carried by
the winds, they reach the stratosphere without being modified.
Here their molecules are broken down by the intense UV light,
and free chlorine atoms are created by this degradation.
Each chlorine atom can destroy several thousands of ozone
molecules before being removed from the atmosphere : chlorine is
a catalist for the ozone depletion . Bromine ( e.g. from methyl
bromide, used by farmers as a fumigant) is even more effective than chlorine.
What are the effects of the reduction
of the ozone layer ?
The reduction of ozone layer will cause an increase of UV
radiation at earth level. An excess of UV rays has been linked
to skin burns, skin cancer, cataracts, and harm to certain crops
and marine organisms.
|
What is being done to stop ozone
depletion?
Replacing the CFCs and other Ozone Depleting Substances with
environmentally safe substances. Researches are going on for
identifying the best alternative substances ; presently HCFCs
(hydrochlorofluorocarbons, substances containing hydrogen,
chlorine, fluorine, carbon) are replacing CFCs, being much less
harmful for the ozone layer. In the future, HCFCs will be phased
out, too.
The "Montreal Protocol" is the 1987 international
treaty governing the protection of stratospheric ozone agreement
to phase out the Ozone Depleting Substances.
According to the Montreal Protocol ( and successive amendments)
usage of the CFCs and most Halons have been reduced or phased out; other ODS, like HCFCs, will be phased out in the
future .
Montreal Protocol was without doubt a great success for the environment, clearly reducing the total amount
of chlorine and bromine entering the atmosphere.
Those reductions should first arrest the decline, then allow the ozone layer to rebuild. Anyway, even if the consumption of all ODS gases would
be completely discontinued, it will take a lot of years
before complete recovering of the ozone layer, due to
their persistence in the atmosphere.
Scientists from NASA and other agencies have developed a new tool - a math-based computer model- to predict
when the timing of ozone hole recovery. The model accurately reproduces the ozone hole area in the Antarctic stratosphere over the past
27 years. Using the model, the researchers predict that the ozone hole will recover in 2068, not in 2050 as currently believed.
"The Antarctic ozone hole is the poster child of ozone loss in our atmosphere," said author Paul Newman, a research scientist at
NASA's Goddard Space Flight Center, Greenbelt, Md.
"Over areas that are farther from the poles like Africa or the U.S., the levels of ozone are only three to six percent below
natural levels. Over Antarctica, ozone levels are 70 percent lower in the spring. This new method allows us to
more accurately estimate ozone-depleting gases over Antarctica, and how they will decrease over time, reducing the ozone hole area."
Notwithstanding some encouraging improvement in the northern mid-latitudes, the anctartic ozone hole has not
yet started to significantly shrink,
something they predict will not start to occur until 2018.
Are greenhouse
gases responsible for ozone depletion?
The most important greenhouse gases ( carbon dioxide,
methane, nitrous oxides ) are not Ozone Depleting Substances .
Anyway the CFCs are greenhouse gases; tropospheric ozone
itself is a greenhouse gas.
Unfortunately some substances used to substitute CFCs, like
HCFCs are less dangerous to ozone, but are greenhouse gases,
too.
|
|



